AstraZeneca’s new manufacturing facility
Pushing boundaries to deliver life-changing medicines AstraZeneca to invest £120 million in new manufacturing facility to produce Zoladex in Macclesfield…
AstraZeneca is a global pharmaceutical company with a major UK presence. Having a global science led biopharmaceutical business and innovative medicines used by millions of patients worldwide.
Zoladex is one of AstraZeneca’s leading cancer drug, supplied to patients across the globe and has been manufactured at AstraZeneca’s Macclesfield plant for over 25 years, this facility is AstraZeneca’s second largest manufacturing site, with over 1800 members of staff processing and distributing medicines to 130 global markets.
As a sterile subcutaneous injectable product, Zoladex is manufactured under highly specialized conditions, which requires a range of high-specification equipment in a controlled environment to ensure aseptic production. The continued global demand for Zoladex has resulted in the decision to invest in replacing some of the existing facilities where the medicine is currently produced.
The building of the new facility began in 2013 and completed in February 2016.
PBSC who are a leading manufacturer of clean room, high containment and material decontamination products around the world provided the expertise and solutions allowing AstraZeneca to continue sterile medicine production.
Products:

Solutions & Results
In association with Boulting Environmental Services Ltd (BES) based in the UK, who help create cutting-edge cleanrooms, laboratories and other sophisticated environments, PBSC contributed with clean room door sets, transfer hatches and vision panels, specially designed for the controlled environment whilst minimizing visible fixings with PBSC’s flush designs.
Main goal: To provide as much vision as possible with the number of vision panels limited due to the low-level extract requirements within this SPP5 facility.
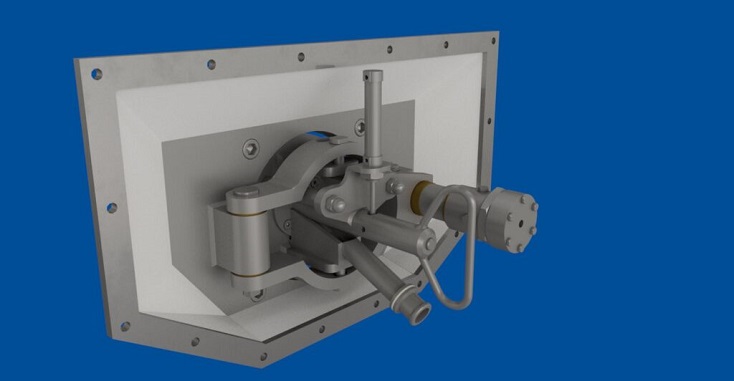
PBSC were the preferred choice, being the only company developing glass doors with concealed automation.
Working closely with BES, PBSC presented a glass door meeting AstraZeneca’s required standard. Having a stainless-steel slider arm which linked to a hygienic stainless-steel circular door rail with a dry running liner bearing, manufactured from water resistant plastic compound.
Thus, removing cleaning issues with access to the motor through the panels on the head fame and having the flush finish which PBSC are famous for.
With the demand for Zoladex which was first launched in 1987 and has become available in more than 100 countries, PBSC with BES, contributed to the continued Zoladex manufacturing, giving a sterile facility the cleanroom products needed to help increase efficiency.
Amanda Tebble – Architectural Design Sales Specialist for PBSC: “I was very happy to be involved in such a globally well-known pharmaceutical company and their new facility”