The ChargePoint Ltd.
is market leader in powder containment and aseptic transfer valves providing operator safety and sterility assurance for the pharmaceutical, biotech, chemical and other process industries.
ChargePoint PharmaSafe®

ChargePoint PharmaSafe® valves ensure the safer handling of Highly Potent Active Pharmaceutical Ingredients (HPAPI) and other formulation ingredients, offering the highest levels of operator protection through validated containment performance.
- Safer handling of non-sterile API and formulation ingredients
- Nanogram level containment performance
- R&D to pilot and production scale formulation
Check out “ChargePoint PharmaSafe” by ChargePoint Technology on Vimeo. The video is available for your viewing pleasure at here.
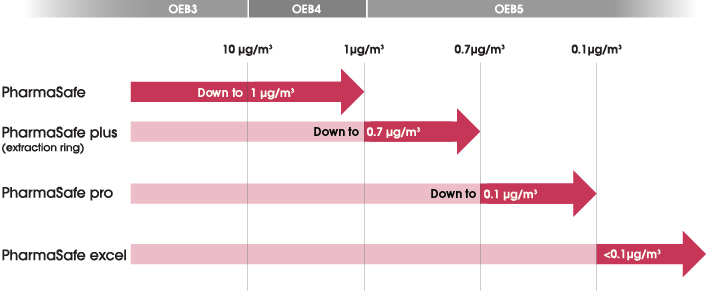
Containment Performance
ChargePoint PharmaSafe® valve containment performance has been independently validated by customers and third parties according to the ISPE SMEPAC (Standardised Measurement of Equipment Particulate Airborne Contamination) guideline.
Benefits
- Ensure industry regulatory compliance.
- Process highly potent ingredients (HPAPI), ensuring the safety of your personnel and reduced environmental contamination.
- Reduce risk of contamination.
- Meet GMP and product quality requirements.
- Maximise yield by transferring poorly flowing and high value product.
- Facilitate respirator free and ‘shirt-sleeve’ manufacturing initiatives by removing costly secondary barrier containment and cumbersome PPE.
ChargePoint AseptiSafe®

Contained Aseptic Transfer Valves
ChargePoint AseptiSafe® aseptic transfer valves offer increased sterility assurance when handling sensitive ingredients and small components in fill/finish aseptic processing and biotech sterile API production.
- Optimise product quality with increased sterility assurance
- Simple in process sterilisation
- High containment performance for hazardous products
Check out “ChargePoint AseptiSafe Bio” by ChargePoint Technology on Vimeo. The video is available for your viewing pleasure at here.
ChargeBag®

High Integrity Single Use Transfer Bags
The contained and sterile transfer of powder and small process components from process to process is now easier than ever with the cost effective ChargeBag®
- Trouble free transfers
- Gamma irradiated for aseptic processing
- Reduce cleaning times
- Economic single use
ChargeBottle® P

Lightweight polypropylene containers to suit a variety of specific process requirements.
ChargeBottle® M

Robust stainless steel / Alloy 22 containers with high quality product contact finish. Non-pressure rated.
ChargeBottle® MX

Pressure rated stainless steel / Alloy 22 containers available with optional purge connections and viewing ports for maximised transfer yield.
Gallery
Related information

Business website:
Relatd industries:
Related Techniques:
Contact Us!
Have any questions? You would like us to call you back?
Send us the following blank sheet. Our staff will contact you soon.














